In late 2023, the Medicines and Healthcare products Regulatory Agency approved Vertex’s Casgevy, which became the inaugural clustered regularly interspaced short palindromic repeats (CRISPR)-based drug for the haematological indications beta thalassemia and sickle cell disease (SCD).
Despite this approval, the CRISPR landscape is dominated by oncology, which accounts for 30% of all active CRISPR drugs.
The precision of CRISPR technology makes it an appealing option for oncology therapeutics, presenting a revolutionary approach to cancer treatment.
On 15 November 2023, Casgevy secured approval for beta thalassemia and SCD treatment in the UK, becoming the first CRISPR drug to receive marketing authorisation globally.
CRISPR technology utilises a complementary RNA sequence to recognise and target specific DNA sequences, working in conjunction with a nuclease protein complex, which cleaves DNA strands.
See Also:
CRISPR technology works with remarkable specificity to rectify non-functional genes, as opposed to replacing or disrupting the pathogenic genes, offering the prevention of disease, instead of a simple treatment.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Thank you!
Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
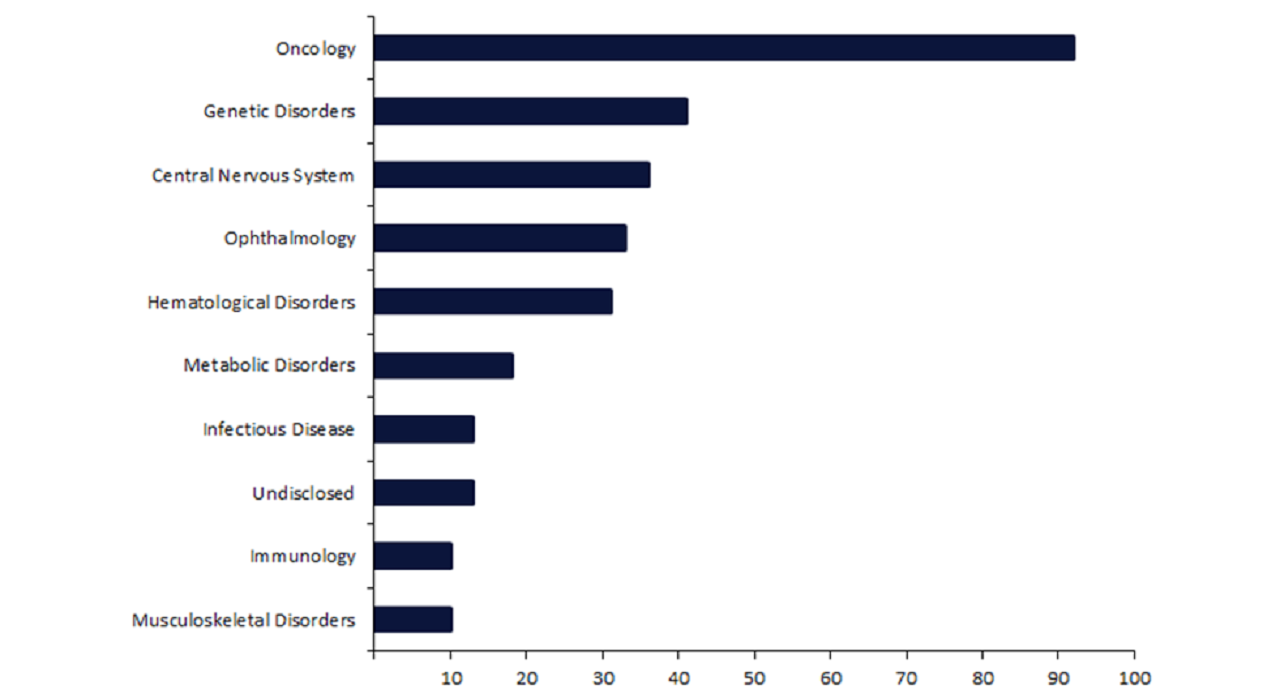
By GlobalDataDespite these historic haematological approvals, this therapy area ranks fifth in terms of the number of CRISPR drugs that are being actively developed or marketed, with only 32 drugs (Figure 1).
In comparison, oncology, featuring 92 drugs, dominates the CRISPR landscape with more than double the second-ranked therapy area, genetic disorders, with 41 drugs, and triple that of haematological disorders.
The focus on oncology could be due to CRISPR’s specificity, which enables a researcher to edit virtually any segment of DNA within the genome, with minimal off-target effects.
With this, CRISPR can be used to target cancer-causing genes and inhibit their function, to impede tumour growth.
Additionally, CRISPR technology can facilitate the engineering of advanced immune cell therapies such as chimeric antigen receptor T-cell (CAR-T) therapies, which have become frontline cancer treatments in recent years.
By eliminating or replacing mutated genes, researchers can enhance the cancer-fighting abilities of these cells, tailoring therapies to individual tumour types and enhancing their ability to recognise and eradicate cancer cells.
CRISPR Therapeutics, the developer of Casgevy, is one of the key players in this space, with 13 oncology drugs in development.
Its lead drug, CTX-110, is a CAR-T therapeutic, modified by CRISPR to enhance the potency of the product.
It is currently in Phase II for various lymphomas and leukaemias, with a global commercial launch by the end of 2025, as estimated by leading data and analytics company GlobalData’s Catalyst calendar.
The high oncology drug count suggests a focus on CRISPR technology within this therapy area.
However, these drugs have not yet progressed beyond Phase II, suggesting that oncology treatments are not advancing more rapidly than those in other areas, and reaffirming the overall immaturity of the pipeline.
Nevertheless, the high specificity of gene editing offered by CRISPR presents a revolutionary approach to treatments and could pave the way for a new era of precise therapies against cancer.






Related Company Profiles
Vertex Inc